
Artificial retina or retina chip is a device that electrically stimulate the retina. The system is based on a pair of glasses with an integrated camera that the patient must wear. The camera sends information to a chip that has been surgically implanted in the retina. This chip sends the information through the optic nerve to the brain.
Taking into account that this system is based on the direct retinal stimulus, it would be suitable for patients suffering from retinal problems, but having a well-functioning, non-damaged optic nerve. In order to send the information to the brain, the optic nerve is essential. This is the reason why it won’t be useful for people who suffer from optic nerve diseases, such as patients with end-stage glaucoma whose optic nerve has already become atrophied. In such cases, this device won’t work.
There are many retinal diseases, but those that could benefit more from such device would be those that affect the most external layers, especially that of the photoreceptor cells. Within these retinal diseases, there is also retinitis pigmentosa.
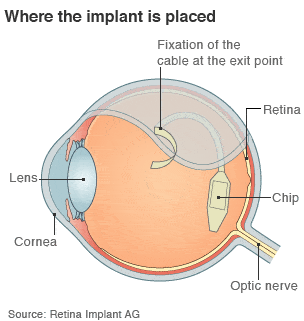
Retina implant
These devices aren’t something new. These systems began to be developed more than 25 years ago. However, results have been disappointing and have not proven this technique to be a proper solution, as the image quality it offers does not have an impact on patient’s quality of life.
When the retina is electrically stimulated, the patient sees flashes or light sparks emitted by each one of the electrodes that the device has. The latest generation systems that have around 150 electrodes generate these sparks on patient’s vision, arranged through his vision field, like 150 pixels of a black and white picture. The image is, thus, very simple.
To get a better idea, current cameras have millions of pixels, so a 150 pixels resolution is a very low image quality. Moreover, these pixels lack of nuances such as color, brightness, contrast, movement and global object perception, which a healthy retina can provide. Humans do not perceive an image which is broke down dot per dot. Instead, when we look at a face, we see the eyes, when we watch a scene, we see a person moving… And that, with a low resolution lacking nuances, which is what offers the chip, it is difficult to interpret.
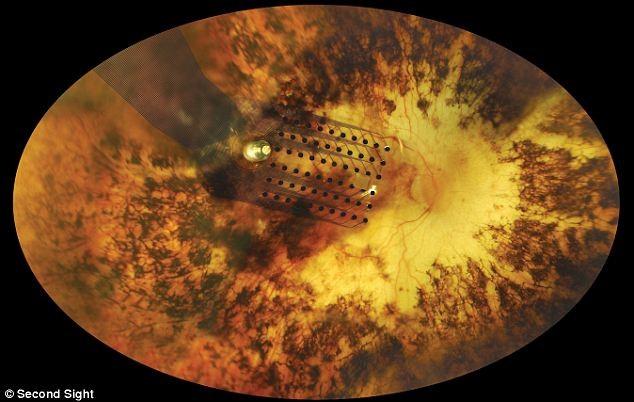
Implanted retina chip
These devices are implanted by means of a surgical intervention implying many risks, both during the intervention and during the postoperative period.
It is difficult to correctly integrate an inorganic material with living tissues, and to stablish a symbiosis that does not alter in any way the homeostasis of such tissues. It is possible that the chip, after being in contact with the retinal surface for a while, generates scarring tissues that may reduce to a substantial extent the transmission of the nervous stimuli. Besides, continuous electrical discharges that stimulate the retina may cause a damaging effect on such tissues, leading to a device failure, and causing the patient to stop seeing those light flashes.
Considerable surgical risks may be taken providing there are subsequent benefits, even remote, for the patient. The issue is that functional results are practically non-existent, so taking those risks does not counterbalance the benefits obtained.
Blindness is due to a malfunction of eye tissues, which are living tissues made up of cells. Any malfunction or cell death in retina leads, therefore to a vision loss. If we aim to restore a patient’s vision, the future lies in trying to recover the function of those cells that are not properly working, or to replace the dead cells for new living cells.
Taking into account that this is regulated by genes that contain the cells, the treatment that aims to recover the cell function is gene therapy. It consists in modifying these genes in order to make the cell recover and work properly.
When a cell is dead and we want to replace it, we need to transplant new cells that may take charge of the function of dead cells. This is what we know as cell therapy.
One of the cell therapies that exist is stem cell therapy. Stem cells are cells present in our body, that have the ability to differentiate themselves in different types of cells. So if we can control the growth of such cells and turn them into retinal cells, they could potentially replace the retinal dead cells.
Stem cells
Both gene therapy and cell therapy are research areas that have achieved huge progresses, not only in ophthalmology, but also in other specialties. I believe we will see major breakthroughs in a near future that will provide an answer to retinal diseases that are currently difficult to treat. We can find an example in the case of Leber’s congenital amaurosis, a rare retinal disease suffered by children. Some encouraging results have been achieved with patients suffering this disease, including a partial vision recovery. The great challenge is to ensure that the benefit for the patient is preserved throughout the time.

Contact us or request an appointment with our medical team.